Raytheon – C-FAST Rapid Covid-19 Diagnostic
Raytheon – C-FAST Rapid Covid-19 Diagnostic
Working with a world-class multidisciplinary team to deliver low-cost, lab-grade COVID-19 testing at home.

The COVID-19 pandemic has raised a global need for tests that are fast, accurate and accessible.
High-complexity lab-based PCR tests are accurate, but getting them to a centralized lab creates delays. Low-cost POC antigen tests are fast and accessible, but not necessarily accurate.
Fast, accurate, and accessible. Pick one — or two, at best. This has been the standard for COVID-19 testing.
Not anymore.


The team at Raytheon BBN Technologies identified that a technology originally designed to detect respiratory disease in cattle could be adapted to detect COVID-19 in humans. Their lateral flow molecular assay-based LAMP test for POC COVID-19 detects the presence of the SARS-CoV-2 RNA in either saliva or nasal swab specimens, without needing to send samples to a lab.
This new solution combined the high accuracy of PCR tests with the low cost of antigen tests, without an invasive nasopharyngeal swab — representing a massive opportunity for increased testing accessibility.
Now it was time to develop a commercially viable product, with a world-class user experience.
Cortex joined a multidisciplinary team including Raytheon, Purdue University, PortaScience, and LaDuca LLC on a remote product development sprint with higher stakes than we had ever seen before.
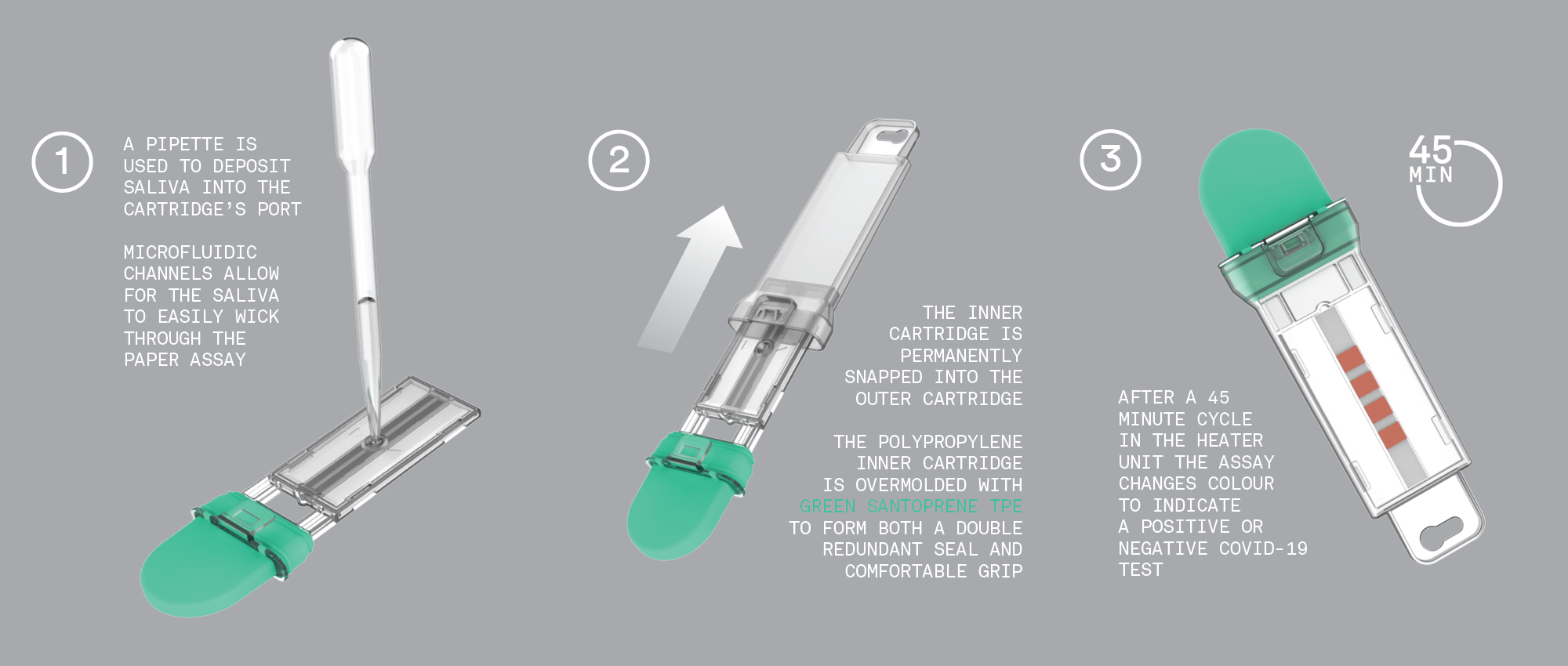
The result is C-FAST — a passive consumable microfluidic cartridge with a simple clinical workflow that delivers accurate test results from a saliva sample in less than an hour, without the use of a lab.
Simplifying the Human Experience
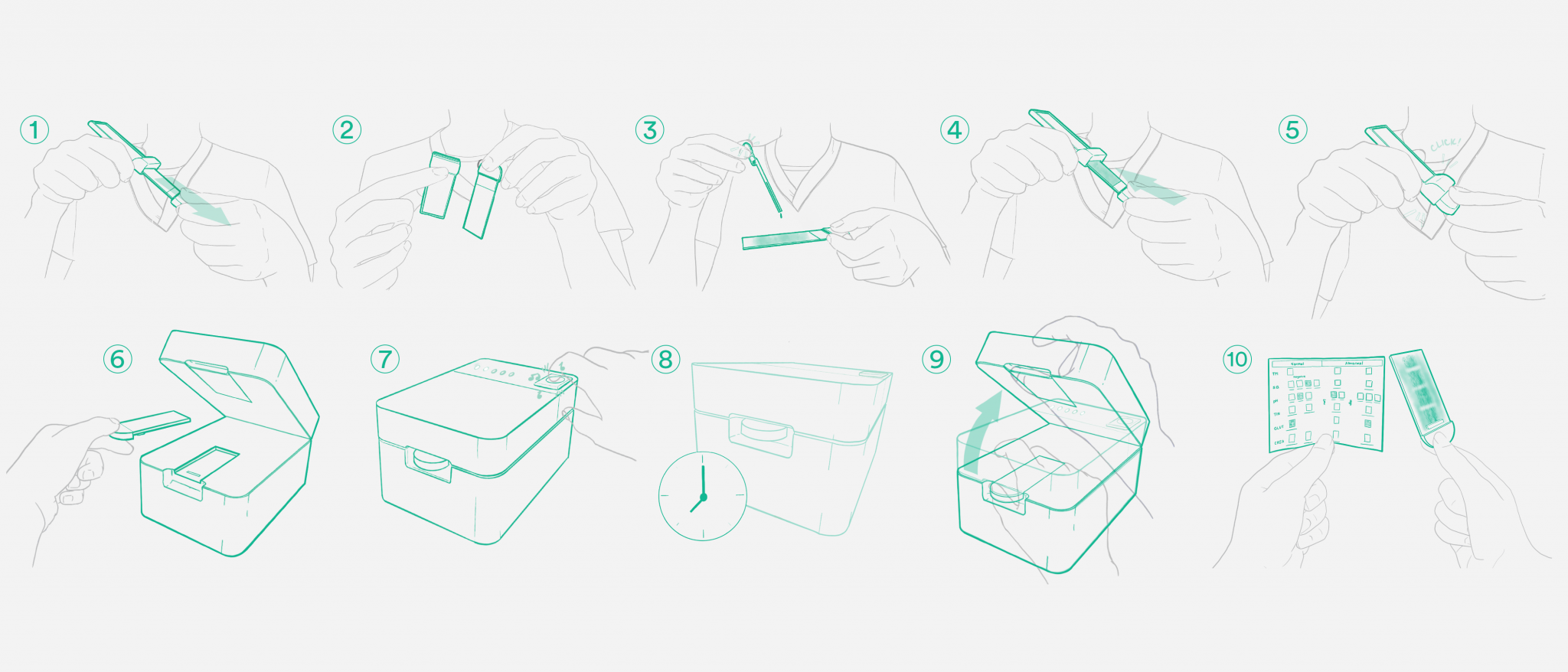
Non-Invasive Sampling With No Clinician
Most COVID-19 diagnostics must be completed by a clinician in order to reduce variations in the test result. This creates bottlenecks for COVID-19 testing and makes it difficult to scale to the general population for ongoing screening — especially in communities with limited access to centralized testing sites.
Simple Clinical Workflow for a Multiplexed Test
The Raytheon methodology enables detection of multiple markers at the same time, potentially enabling simultaneous testing for COVID variants or Influenza A and Influenza B. The team’s philosophy was aligned: design a system that removes complexity from the user, so the human interaction with the product can be simple. We identified the variations that occur with the way a human interacts with the cartridge and designed an embedded system that would dynamically adapt to provide consistent results at the end of the test.
Microfluidic and Thermal Engineering Matters
Aliquoting, sampling, and transport of saliva samples through a microfluidic chamber must be repeatable and reliable over a large number of tests. Expertise in microfluidics is essential in order to achieve consistent results.

Power to the People
The system is designed from the beginning to use low-cost components with sophisticated mechanical and thermal engineering to do the heavy lifting. The result is a system with lab-based accuracy at a cost a general consumer can afford to do continuous COVID-19 screening at home or at work.


Cortex Fundamental Deliverables
- Concept development
- Functional prototype development
- Custom circuit board design
- Microfluidic design and functional prototyping
- Test validation
- Regulatory Compliance oversight
- Ongoing support for contract manufacturing
This work is owned by Raytheon BBN Technologies Corp. If you are interested in licensing this technology, please contact Susan Katz at (617) 873-2339 or susan.katz@raytheon.com.

Work / Medical
Neursantys
Pioneering the discovery and delivery of bioelectronic treatments using non-invasive devices to target neuromotor conditions that have limited treatment options.
Work / Medical
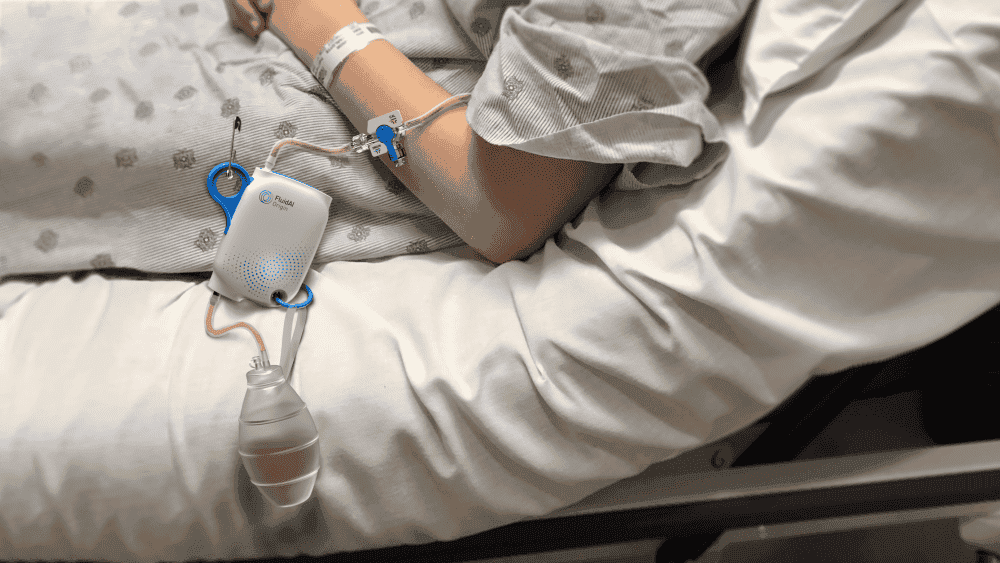
FluidAI- Origin
Design-led development of FluidAI’s non-invasive postoperative infection monitoring platform.
Work / Commercial
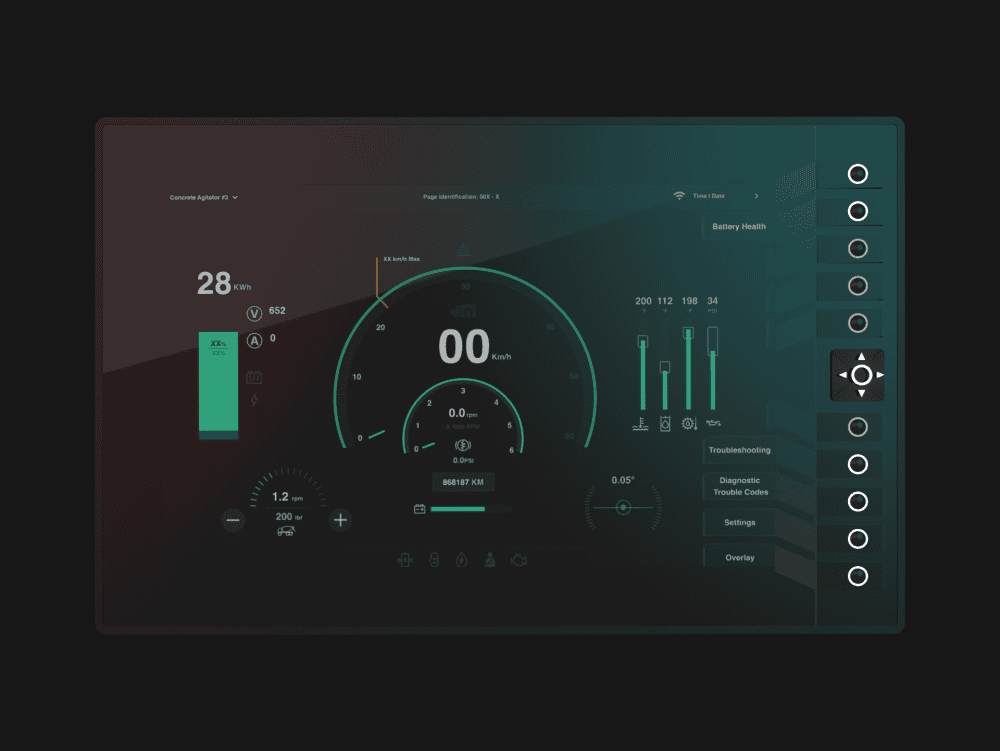
Maclean Engineering
Entirely re-thought user interface design for the next generation of electric underground mining equipment